
The iron that flows out of a blast furnace contains large amounts of carbon, left over from the coke, and several other impurities. To transform this iron into steel, the excess carbon and the impurities must be removed.
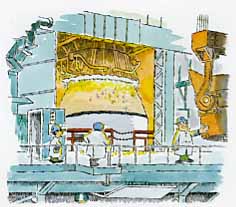
The basic oxygen furnace, or BOF, offers an efficient, economical way of accomplishing this metallurgical trick. J&L's BOFs at Aliquippa, Cleveland and Indiana Harbor refine the major portion of the steel we make.
A BOF is nothing more than a pear shaped metal bowl lined with a special chemically active heat-resistant brick. The word "basic" in BOF refers to the brick's chemistry, not to the simplicity of a BOF's operation.
Making steel in a BOF involves a five-step process:
1. The BOF is tilted sideways and is charged with a partial load of steel scrap.
2. Molten iron from the blast furnace is added on top of the scrap. Typically, the iron represents 70 per cent of the metal in the vessel.
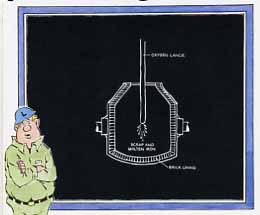
3. The BOF is turned upright and a water-cooled oxygen lance is inserted into its opening. The lance shoots a high-pressure stream of nearly pure oxygen against the charge's surface (above). The oxygen-induced chemical reactions cause a rapid heating that completely melts the scrap and brings the charge to proper refining temperature. The turbulence of the oxygen stream stirs and agitates the molten metal.
4. While the oxygen "blow" continues, burned lime and other fluxing agents are added. The oxygen burns away the excess carbon and many of the impurities; the slag formed by the fluxing agents captures the rest.
5. At the end of the blow certain elements, manganese, aluminum, silicon and sulfur, for example, may be added to the molten steel to produce specific properties.
Making a 200-ton batch of steel in a BOF takes about 45 minutes... including the 15-minute oxygen blow.