

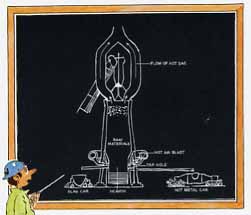
A blast furnace gets its name from the way it works: in operation, a blast of hot air blows upward through the molten ingredients inside the furnace.
When you get down to basics, a blast furnace is little more than a tall metal chamber lined with special heat resistant bricks (above).
Raw materials are fed into the top of the furnace and gradually work their way down to the bottom. In the process they are heated to over 3000"F; they melt, and chemically react together to liberate molten iron from the iron ore.
Interestingly, once a blast furnace is fired up, or blown-in, it runs continuously until the heat-resistant bricks need replacing. J&L's blast furnaces at Aliquippa, Cleveland, Pittsburgh, Youngstown and East Chicago often operate for years at a stretch without being shut down.
Iron ore, coke and limestone are the primary raw materials dropped into a blast furnace. On the average, 3300 pounds of ore, 1200 pounds of coke and 500 pounds of limestone are needed to produce 2000 pounds of iron. The coke performs two vital functions:
1. It burns in the blast of hot air to heat the furnace to operating temperature, and
2. It generates large volumes of carbon monoxide gas as it burns. This gas is the chemical agent that strips away the oxygen in the iron ore, leaving a pool of molten iron in the bottom of the furnace.
The limestone is present to form a layer of molten slag on top of the molten iron that traps and holds impurities that were present in the ore and coke.
Periodically, the blast furnace is tapped to remove the molten slag and the molten iron.
The blast of hot air is produced by powerful
blowers that push cold air through a series of tall cylindrical
structures called stoves. Gas, produced at the top of the blast
furnace, is burned in the stoves to heat the incoming air to about
2000°F. The excess gas produced by the blast furnace is burned
in boilers to generate steam. This makes the blast furnace one
of industry's most energy efficient production units.