

Metallic iron loves to combine with oxygen. Consequently, pure iron is almost never found in nature. Iron ores are essentially compounds that contain both iron and oxygen.
Until quite recently, only natural or red iron ore was suitable for steelmaking. This ore contains a relatively high concentration of iron and is relatively easy to concentrate.
Natural ore used today is found in deposits that are fairly close to the surface. Once the earth and rock overburden has been removed to expose the ore, the ore is scooped up by giant shovels, then trucked to a processing plant to be screened and concentrated.
Natural ore, after concentration, can be fed directly into a blast furnace to make iron. It is the simplest ore to use. However, deposits of natural ore are rapidly being exhausted. Consequently, the steel industry has developed ways to use low-grade ore that is available in abundant quantities.
Actually, deposits of natural ore are like
the raisins in a dish of rice pudding: they are surrounded by
deposits of low grade ore.
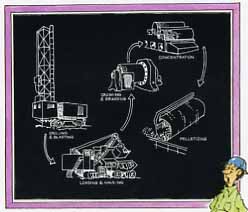
This low-grade ore is called taconite; it is an incredibly hard rock that contains a relatively low concentration of iron. Taconite cannot be used in a blast furnace as it comes from the ground.
Instead, taconite ore is first crushed and ground to a powder-like consistency (above). The iron-containing particles are separated from the worthless rock by magnetic concentration or flotation. Finally, these iron-rich particles are formed into small pellets, about as big as marbles, that are suitable as blast furnace feed.
Though the process is expensive, the pellets have a higher iron content than natural ore and, thus, increase the iron production rate in the blast furnace while using less energy. This partially offsets the high cost of the pelletizing operation.
AL receives 10 million tons per year of
pellets from iron mines in Michigan's Upper Peninsula, Minnesota,
and Canada that we own jointly with other companies.