
Because iron has a great affinity for oxygen, an unprotected steel surface will tend to combine with oxygen in the air to form iron oxide. This is another way of saying that it will rust. One way to help protect steel from corrosion is to coat its surface with thin coatings of other metals that do not combine readily with oxygen. Zinc and tin are the two most widely used metals. Zinc-coated steel is called galvanized steel; tin coated steel is called tin plate.
An important point to keep in mind is that this coating of tin or zinc does not simply cling to the steel surface like paint. Instead, the bottom of the zinc or tin layer actually forms an alloy with the steel surface to create a durable protective barrier.
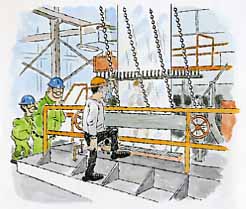
Galvanized steel has numerous applications, including car body components, buckets and tubs, air conditioning ductwork and water storage tanks. J&L galvanizes steel sheet products at our Hennepin, Pittsburgh, and Indiana Harbor works via the hot-dip process. In this process, carefully cleaned steel sheet is annealed to give it desirable mechanical properties and then drawn through a bath of molten zinc (above left). The coated sheet is allowed to cool slowly, giving the molten zinc sufficient time to alloy with the steel surface. Finally, the sheet is rolled into coils or cut into flat sheets.
The major application of tin plate is the manufacture of "tin cans" for food and beverages. Actually, the familiar name is a misnomer: a tin can is 98 per cent steel and only two per cent tin.
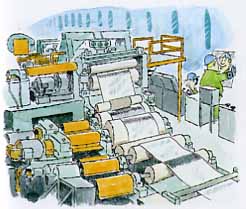
Tin is applied to the surface of steel sheet by an electrolytic process. J&L's tin mills at our Aliquippa and Indiana Harbor works first roll hot rolled bands to make cold rolled sheet. Then, the tin layer is applied. Here's how:
The surface of the steel sheet is carefully
cleaned and rinsed. Next, a solution that can conduct electricity,
called an electrolyte, is applied to the surface and the sheet
is drawn past in electrodes. An electric current flowing between
the sheet and the electrodes deposits tin on the sheet's surface.
Finally, the tin plate is heated to melt the tin and create a
smooth, shiny surface (above).