

Stainless steel contains lots of chromium and nickel, expensive metals that are in short supply. Consequently, J&L's Warren Plant makes stainless steel by a two-step process that prevents needless wastage of chromium.
Step 1: Carefully selected stainless steel scrap is melted in an electric furnace along with chrome-containing alloys and slag-forming flux materials. The steel is partially refined during this step.
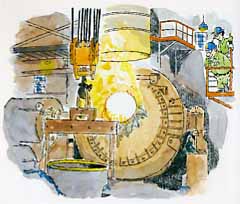
Step 2: Molten steel is transferred to an argon-oxygen decarburization vessel (AOD vessel) for final refining (above).
Don't let this technical-sounding mouthful throw you; an AOD vessel looks and works much like a basic oxygen furnace, with two major differences:
1. A mixture of argon and oxygen gas rather than pure oxygen, is used to burn away the excess carbon in the molten metal. Decarburization" is a technical way to say "remove carbon:'
2. The gases are blown up through the bottom of the vessel; an external lance is not used (above).
The purpose of the argon is to help prevent the loss of chromium. Simply stated, the argon reduces the amount of chromium that is burned off along with carbon.
At the start of the gas blow, more oxygen is used than argon, but as the process continues, oxygen is gradually replaced with argon. The relative proportions of the two gases must be carefully controlled at all times. The molten metal spends about two hours in the AOD vessel.
Our Warren Plant does not manufacture finished stainless steel products. Instead, it produces stainless steel slabs that are rolled at our Cleveland Works and Louisville, Ohio, plant.
Stainless steel is a specialty product,
both in the ways it is used and made. Our Warren Plant makes only
about 25 tons of stainless steel each hour. Contrast this with
the more than 400 tons an hour of carbon steel we can make at
Aliquippa.